External dental implants

External dental implants
External dental implants
The External dental implant, originally known as the Maestro, has performed successfully in every published study since its launch in 1997. The External implant has survival rates from 98.5% to 100% in studies of up to 1,400 implants and a follow up of 10 years.1-10 It has also been shown to achieve bone-to-implant contact levels of 80.6%.11 Clinicians confidently rely on the External implant to achieve consistent, reproducible results necessary to build a successful implantology practice.
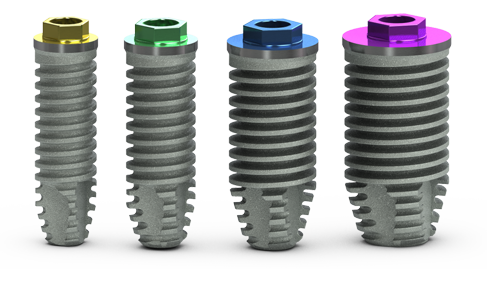
features
square threadform
restorative ease

mounted configurations
- Mounted with 3inOne abutment

full range of sizes

specifications13
- parallel wall
- external hex
- supercrestal
- crestal
- RBT body
- HA body
- 3inOne Abutment
- 3.5mm
- 4.0mm
- 5.0mm
- 6.0mm
- 3.5mm
- 4.0mm
- 5.0mm
- 6.0mm
- 7mm
- 9mm
- 10.5mm
- 12mm
- 15mm
additional information
- Posterior Implant Single-tooth Replacement and Status of Adjacent Teeth during a 10-year Period: A Retrospective Report.
Misch CE, Misch-Dietsh F, Silc J, Barboza E, Cianciola EJ, Kazor C. J Periodontol. 2008 Dec;79(12):2378-2382. - A Bone-Quality Based Implant System: First Year Prosthetic Loading.
Misch CE, Dietsh-Misch F, Hoar J, Beck G, Hazen R, Misch CM. J Oral Implantol. 1999;25(3):185-197. - A Prospective Multi-Center Clinical Investigation of a Bone Quality-Based Dental Implant System.
Kline R, Hoar JE, Beck GH, Hazen R, Resnik RR, Crawford EA. Implant Dent. 2002;11(3):224-234. April 30-May 4, 1997. New Orleans, LA. - Initial Clinical Efficacy of 3-mm Implants Immediately Placed into Function in Conditions of Limited Spacing.
Reddy MS, O'Neal SJ, Haigh S, Aponte-Wesson R, Geurs NC. Int J Oral Maxillofac Implants. 2008 Mar-Apr;23(2):281-288. - Immediate Functional and Non-functional Loading of Dental Implants: A 2- to 60-month Follow-up Study of 646 Titanium Implants.
Degidi M, Piattelli A. J Periodontol. 2003 Feb;74(2):225-241. - Five-Year Prospective Study of Immediate/Early Loading of Fixed Prostheses in Completely Edentulous Jaws with a Bone Quality-Based Implant System. Misch CE, Degidi M. Clin Implant Dent Relat Res. 2003 May;5(1):17-28.
- Comparative Analysis of Immediate Functional Loading and Immediate Nonfunctional Loading to Traditional Healing Periods: A 5-Year Follow-Up of 550 Dental Implants.
Degidi M, Iezzi G, Perrotti V, Piattelli A. Clin Implant Dent Relat Res. 2008 Sep;9. - Endosteal Implants in the Edentulous Posterior Maxilla: Rationale and Clinical Report.
Misch CE, Poitras Y, Dietsh-Misch F. Oral Health. 2000 Aug;8:7-16. - Augmentation of the Maxillary Sinus with Calcium Sulfate: One-Year Clinical Report from a Prospective, Longitudinal Study.
De Leonardis D, Pecora GE. Int J Oral and Maxillofac Implants. 1999 Nov-Dec;14(6):869-878. - Short Dental Implants in Posterior Partial Edentulism: A Multicenter Retrospective 6-year Case Series Study.
Misch CE, Steignga J, Barboza E, Misch-Dietsh F, Cianciola LJ, Kazor C. J Periodontol. 2006 Aug;77(8):1340-1347. - Histologic and Histomorphometric Findings from Retrieved, Immediately Occlusally Loaded Implants in Humans.
Romanos GE, Testori T, Degidi M, Piattelli A. J Periodontol. 2005 Nov;76(11):1823-1832. - Functional Surface Area: Thread-Form Parameter Optimization for Implant Body Design.
Strong J. Todd, Misch Carl E., DDS, MDS, Bidez Martha W., PhD, Prasad Nalluri. Compendium / Special Issue. Vol 19, No. 3, 1998. - Implants are not available in all platform diameter, body diameter and length configurations. Please visit store.biohorizons.com for full availability.








